Part Of: Demystifying Physics sequence
Content Summary: 700 words, 7min reading time.
Energy As Universal Currency
Why does burning gasoline allow a car to move? Chemical reactions and kinetic propulsion seem quite distinct.
How does a magnet pull a nail from the ground? What relation exists between magnetism and gravitational pull?
What must occur for a nuclear reactor to illuminate a light bulb? What connection is there between nuclear physics and light waves?
Energy is the hypothesis of a hidden commonality among the above phenomena. There are many forms of energy: kinetic, electric, chemical, gravitational, magnetic, radiant. But these forms are expressions of a single underlying phenomena.
A single object may possess many different energy forms simultaneously:
A block of wood thrown into the air will possess kinetic energy because of its motion, gravitational potential energy because of its height above the ground, chemical energy in the wood (which can be burned), heat energy depending on its temperature, and nuclear energy in its atoms (this form is not readily available from our block of wood, but the other forms may be).
Non-physicists worry that physics involves memorizing a giant catalogue of phenomena, each discovered by some guy who got an equation named after him. Energy is the reason why physics is not “stamp collecting”. It allows us to seamlessly switch between different phenomena.
Change As Energy Transformation
The only thing that is constant is change.
-Heraclitus
Certain Greek philosophers were obsessed with change. Physics gives us a language that formalizes these intuitions. Consider the following.
- Energy is the capacity to do work; that is, initiate processes.
- Processes (i.e., work) involve continuous and controlled actions, or changes.
A confusing diversity of phenomena can produce force (the ability to accelerate things), but they all share the same blood. Change is energy transformation.
We can play “where does the energy come from” game on literally any event in the physical universe:
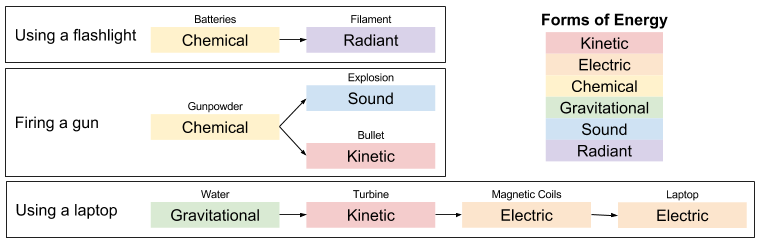
A Worked Example
Let’s get concrete, and go through a simple illustration of work as energy transformation.
Our example involves a ball sliding down an incline.
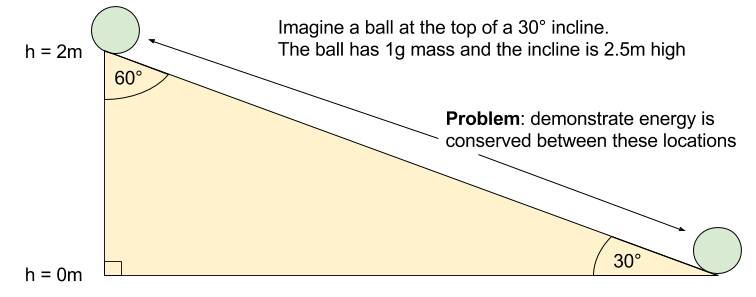
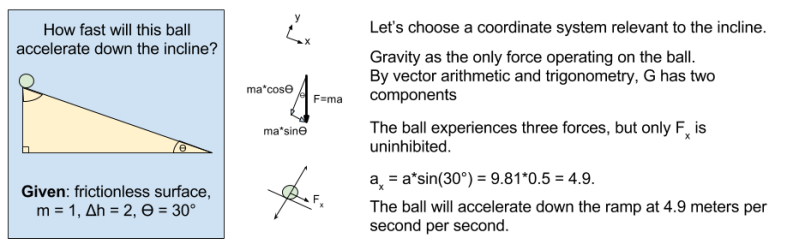
To get our bearings, let’s calculate the acceleration experienced by the ball
To demonstrate conservation of energy, we first need to solve for the ball’s final velocity (v) at the bottom of the ramp. Recall the lesson of kinematic calculus, that displacement, velocity, and acceleration are intimately related:
We can use these formulae to calculate final velocity (for a tutorial see here).
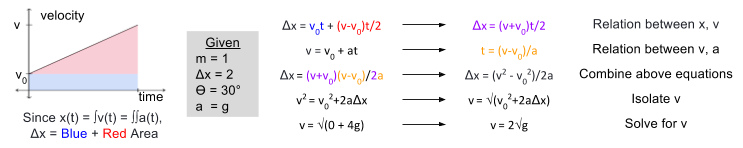
So the ball’s final velocity will be meters per second
m/s). However, recall the classical definitions of kinetic and potential (gravitational) energy, which are
and
.
If conservation of energy is true, then we should expect the following to hold:
Is this in fact the case? Yes!
Total energy of the ball stays the same across these two points. Conservation of energy can also be demonstrated at any other time in the ball’s journey. In fact, we can show that potential energy is smoothly converted to kinetic energy (image credit Physics Classroom).
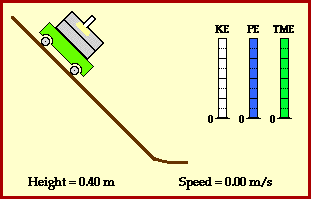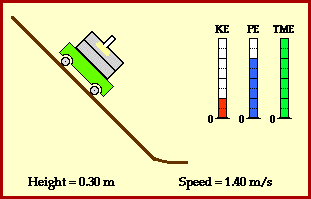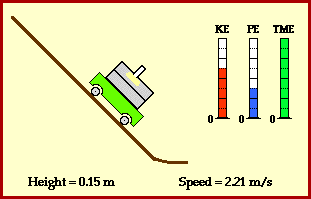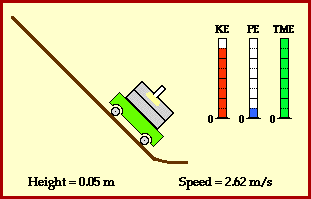
Why is the ball transforming its energy ? Because it is experiencing a force.
Cosmological Interpretation
Just as space and time are expressions of a single spacetime fabric, Einstein also demonstrated mass-energy equivalence. Mass is simply a condensed form of energy, capable of being released in e.g., nuclear explosions. Do not confuse mass and matter. Mass and energy are (interchangeable) properties of matter.
So there are two components of reality: energymass and spacetime. Spacetime bends for energymass. Energy is conserved, but has many faces. The laws of physics (quantum field theory and general relativity) describe the interactions between energymass and spacetime. And since we know that energy is conserved, all there is today was all there was at the beginning of time.
If the amount of energy contained in our universe doesn’t change, what is this quantity? How much energy is there? One strong candidate theory is zero. The flat universe hypothesis rests on the realization that gravitational energy is negative, and claims that it counterbalances all other mediums.
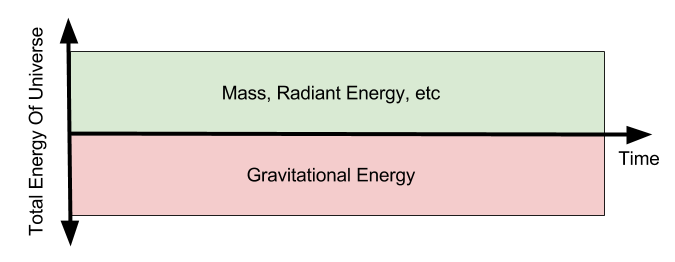
Next time, we will explore the distinction between usable vs inaccessible energy. Until then.
Curious about the direction this sequence goes. I’d be really interested in seeing a discussion of how (or if) the concept of usable energy (or free energy) converges with information theory.
LikeLike
Thanks for your comment Travis! I am indeed moving in that direction (see my latest post).
The structural form of Shannon entropy (information theory) and Boltzmann entropy (thermodynamics) are indeed embarrassingly similar, and we are just understand the ramifications of this identity.
A couple recommendations if you are impatient:
Jaynes: “Information Theory and Statistical Mechanics”
Baez: https://johncarlosbaez.wordpress.com/2010/10/12/algorithmic-thermodynamics/
Friston: https://en.wikipedia.org/wiki/Free_energy_principle
LikeLike
I’ll be watching with interest. The Baez post was an interesting read. I wish I had a better handle on everything in this domain. Whenever I explore this topic I get the sense that I’m just nibbling on the crumbs of what is actually a royal banquet – that is to say, it seems that there are far-reaching implications to the ideas in this domain that I have yet to fully appreciate.
LikeLike